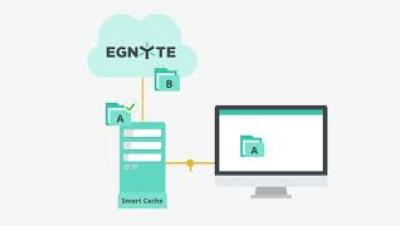
Egnyte and Splunk Integration: You Can't See if You Don't Look
Security Information and Event Management (SIEM) technology provides visibility across an organization's information security systems by collecting and correlating events from logs across many different sources. Security analysts use tools like a SIEM to go “threat hunting”. By correlating disparate events across systems, they can often detect Indicators of Compromise (IoC’s) that may otherwise go unnoticed on individual systems.